GTPases
G-proteins, guanine nucleotide binding proteins, are membrane-associated trimeric proteins that associate with receptors to participate in cellular signaling. Large G-proteins comprise the GDP/GTP-binding α-subunit, which is asssociated with the βγ-subunits. Small monomeric G-proteins like Ras are important molecular switches that also participate in signal transduction pathways.
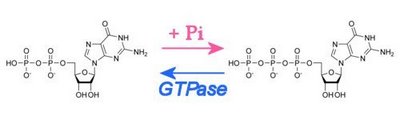 _______________GDP________________________ GTP
_______________GDP________________________ GTP In association with a GPCR, ligand-binding by the transmembrane receptor induces the α-subunit to exchange its bound GDP molecule for a GTP molecule, upon which the G-protein dissociates from the βγ-subunit and the receptor. The receptor is now free to engage another G-protein trimer, and both the α-GTP- and βγ-subunits are free to activate signaling cascades (second messenger pathways), gate ion channels, and activate effector proteins. As a GTPase, the α-GTP-subunit hydrolyzes its attached GTP to GDP, freeing the α-subunit to re-associate with the βγ-subunit and the receptor, initiating a new cycle. Thus, the G-protein acts as both an amplifier and a transducer of the signal.
GTPase superfamily functions:
a. Signal transduction at the intracellular domain of transmembrane receptors (GPCRs), including sensory perception (taste, smell, light).
b. Protein assembly (translation) at ribosomes.
c. Regulation of cell cycle, and cellular differentiation.
d. Active transport and transmembrane protein transport (translocation).
e. Transport of vesicles within the cell, where GTPases control the assembly of vesicle coats.








































