serine proteases
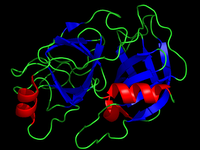 Serine proteases, now renamed serine endopeptidases are peptidases (formerly proteases) that cleave peptide bonds in target proteins. Serine endopeptidases (proteases) are so-named for the invariant presence of a serine residue in the active site. (left - trypsin)
Serine proteases, now renamed serine endopeptidases are peptidases (formerly proteases) that cleave peptide bonds in target proteins. Serine endopeptidases (proteases) are so-named for the invariant presence of a serine residue in the active site. (left - trypsin)▼ actions : clans : coagulation : complement : cytotoxicity : digestion : evolution : families : serine protease inhibitors : serpins ▼
In mammals, serine proteases function in coagulation, immune and inflammatory responses, and act as digestive enzymes in both prokaryotes and eukaryotes:
● cytotoxic activity of cytotoxic lymphocytes (NK cells, killer T cells)
● digestion – chymotrypsin, trypsin, elastase
● blood clotting – Factor 10 (X), Factor 11 (XI), thrombin, plasmin
● complement system – C1r and C1s, C3 convertases ( C3/C5, C5, C4b.2b, C3b, Bb)
Serine proteases (endopeptidases) are subdivided into
● clans that share structural homology,
then further subgrouped into
_ ● families that share close sequence homology.
In humans, clans of serine endopeptidases are:
● chymotrypsin-like clan – chymotrypsin, elastase, trypsin
● subtilisin-like clan – prokaryotic enzyme employing an evolutionarily convergent catalytic mechanism utilizing a catalytic triad to create a nucleophilic serine that attacks the carbonyl carbon of the scissile peptide bond of the substrate
● α/β hydrolase clan
● signal peptidase clan
Serine protease inhibitors (serpins) are a diverse group of molecules that form a covalent bond with the serine protease, irreversibly inactivating endopeptidase function. Serpins include antithrombin, alpha 1-antitrypsin,and alpha 1-antichymotrypsin.
▲♦ : actions § alpha 1-antichymotrypsin § alpha 1-antitrypsin ♦ bonds : clans : coagulation § coagulation factors : complement ♦ covalent bond : cytotoxicity : digestion : evolution : families : serine protease inhibitors : serpins ▲♦
tags [Proteins] [acute phase] [alpha-1 antitrypsin] [alpha-1 antichymotrypsin] [antithrombin] [inflammation] [complement] [serine protease inhibitor]








































